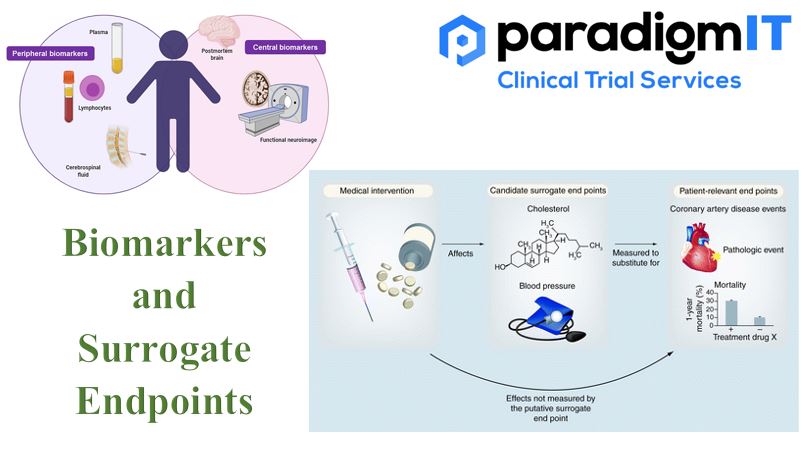
Bio-markers have versatile applications in healthcare, from diagnosis to prognosis. Apart from indicating the changes in physiologic and pathological health, they help us to understand the bodily response to different therapeutic interventions and measure the specific outcomes. As part of the clinical research continuum, biomarkers can help in the recruitment of clinical trial participants, the real-time monitoring of progress during the trials and inferencing the clinical outcomes. Biomarkers can be molecular or histologic, or radiographic. They are also categorized based on their applications (predictive, prognostic etc.).
Types of Biomarkers:
- Diagnostic biomarkers – used to identify the presence of a disease or condition. For example, a genetic mutation associated with a particular disease.
- Prognostic biomarkers – to predict the likelihood of disease progression or outcome. Tumor markers may be used to predict the likelihood of cancer recurrence is an example.
- Predictive biomarkers – to calculate response to a particular treatment. Example: HER2/neu expression in breast cancer patients predicts response to trastuzumab therapy.
- Monitoring biomarkers – used to track disease progression or treatment response over time. Blood glucose levels in diabetic patients are a monitoring biomarker.
- Safety biomarkers – to monitor the safety of a treatment. For example, liver enzyme levels can indicate liver damage caused by a medication.
- Pharmacodynamic biomarkers – to assess the biological activity of a drug. Blood pressure reduction is a pharmacodynamic biomarker for antihypertensive medications.
Surrogate Endpoints (SEs), in basic terms, are substitutes for measuring clinical outcomes in a clinical trial. SEs are particularly useful when the clinical outcomes take longer to study (such as in Stroke). Sometimes, clinical researchers may want to understand the clinical improvement of a particular SE (like Blood Pressure) as an overall indicator of the clinical outcome of the study. Biomarkers (such as laboratory measurements) can also be used as SEs to predict the clinical benefit of therapeutic interventions.
One needs to be cautiously optimistic while considering SEs as a substitute for clinical outcomes. Because SEs need to have extensive clinical evidence before they can be reliably applied to decipher a clinical benefit.
Types of Surrogate Endpoints:
- Biomarker Surrogate Endpoints – are based on measurable biological markers that are correlated with clinical endpoints. For example, blood pressure can be used as a surrogate endpoint for cardiovascular disease.
- Imaging Surrogate Endpoints: used to measure changes in disease progression, based on imaging techniques, such as MRI or CT scans. Tumour size can be used as a surrogate endpoint for cancer survival.
- Physiological Surrogate Endpoints: to predict clinical endpoints, based on physiological measurements, such as heart rate, Example: changes in cholesterol levels can be used as a surrogate endpoint for cardiovascular disease.
- Pharmacodynamic Surrogate Endpoints: These endpoints are based on the effects of a drug on the body, such as changes in enzyme activity or hormone levels. Changes in blood glucose levels can be used as a surrogate endpoint for diabetes.
- Patient-Reported Surrogate Endpoints: These are based on patient-reported outcomes, such as pain levels or quality of life, that are correlated with clinical endpoints. For instance, changes in pain levels can be used as a surrogate endpoint for arthritis.
It is important to note that surrogate endpoints should be validated before they are used in clinical trials. This involves demonstrating that the surrogate endpoint accurately predicts the clinical endpoint, and that the drug has a significant effect on the surrogate endpoint. Based on the level of validation available, following are the different stages of development in the process of validating surrogate endpoints.
Validated SEs:
Accepted by FDA as they are strongly validated to correlate with clinical outcome/benefit.
Classic Examples: Low-Density Lipoprotein Cholesterol (LDL) level (Validated SE) and Occurrence of Heart attack (Correlated Clinical Outcome)
Reasonably likely SEs
The rationale is strong, but they cannot be used standalone to correlate with clinical benefits.
More clinical data may be required.
Candidate SEs
They have the potential to be studied for their ability to correlate with clinical benefits.
One needs to understand that the application of SE is always contextual, and it differs on a case-to-case basis. Biomarkers and SEs don’t always give the full picture about the benefits and risks of clinical intervention. To counter that, Biomarker SEs can also be a part of a composite endpoint where it is used in combination with clinical end points or clinical outcome assessment. Despite current challenges with validating the biomarkers and SEs, they are still expected to play a vital role in the future of clinical trials and medical product development.
For more information –
Visit our website – www.paradigmit.com
Or you can write us at ask@paradigmit.com
Follow us for more – https://www.linkedin.com/company/paradigmittechnologyservices/?viewAsMember=true