Even with all the modern-day innovations in clinical research, Pediatric Clinical trials still present an ethical and legal dilemma due to the concerns regarding the well-being of the children participants involved. The scientific merit of the clinical study protocols and expected positive outcomes need to be justifiably better than the level of risk exposure to […]
CDISC stands for Clinical Data Interchange Standards Consortium, and SDTM stands for Study Data Tabulation Model. CDISC is the organization that develops a set of universal standards to support crucial activities such as data acquisition, exchange, submission and archival in clinical research, benefiting all the stakeholders involved (such as researchers, technology vendors and pharma companies) […]
Clinical Data Archiving and Retention are important components of the “Close Out” phase of Clinical Data Management. Regulatory requirements mandate the retention of all records and data collected during clinical trials. In addition to retaining the data, there is also a requirement to archive all the data in a single platform for a specified period. […]
Data quality is crucial for success in the world of clinical trials, where accuracy and reliability are crucial. Data quality metrics serve as a guiding light, aiding scientists, physicians, and other stakeholders in making informed decisions and pioneering advancements in research. Poor data quality can lead to inaccurate results, which can have serious consequences for […]
Version control and change management are essential practices for ensuring the integrity and accuracy of clinical data. By tracking changes to data over time, version control allows you to revert to previous versions if necessary. Change management ensures that changes to data are made in a controlled and documented manner so that you can track […]
In the realm of clinical trials, Data Visualization and Business Intelligence (BI) have emerged as powerful tools, catalyzing informed decision-making and accelerating research outcomes. Data visualization and business intelligence (BI) are important techniques for increasing the efficiency and effectiveness of clinical studies. Data visualization can assist researchers in identifying trends, patterns, and outliers that would […]
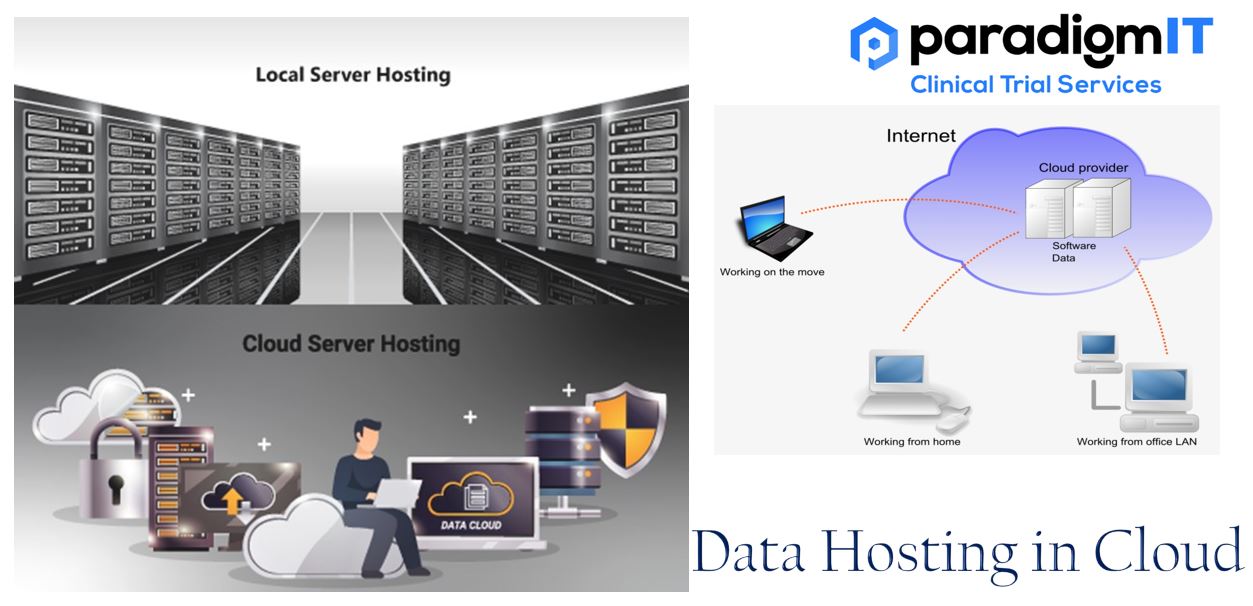
In the field of clinical trials, adopting cloud-based data hosting provides a game-changing advantage. This method modernizes data management by improving security, flexibility, and efficiency. The cloud is a powerful tool that can be used to store and manage data for clinical trials through the internet. This infrastructure eliminates the need for physical servers and […]
Ever wondered how crucial information travels seamlessly in the world of clinical trials? It’s like a digital adventure powered by Data Export and Transfer – a process that might sound complex but holds the key to unraveling medical mysteries and advancing healthcare.Data is the heartbeat of decision-making in today’s digital landscape, and its seamless flow […]
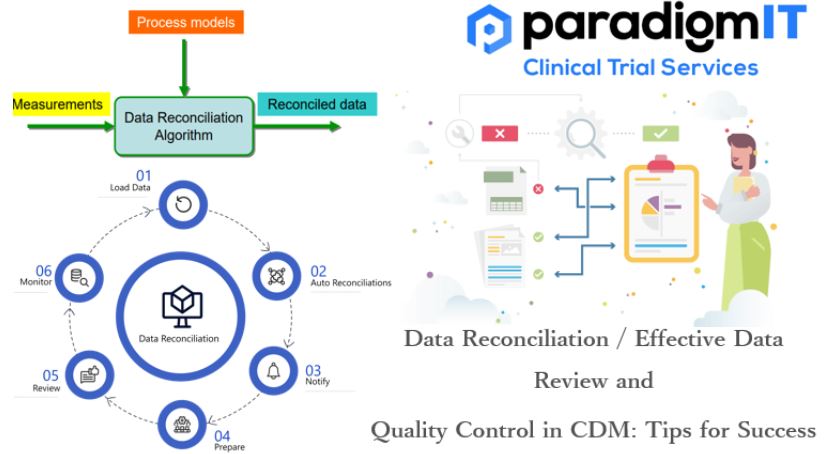
Clinical Data Management (CDM) is a continuous process and a crucial component of clinical trials. CDM starts with designing the case report forms and database design in the first stage and goes on to data capture, data cleaning, data reconciliation, medical coding, and data validation in the second stage. In the third stage, clean data […]
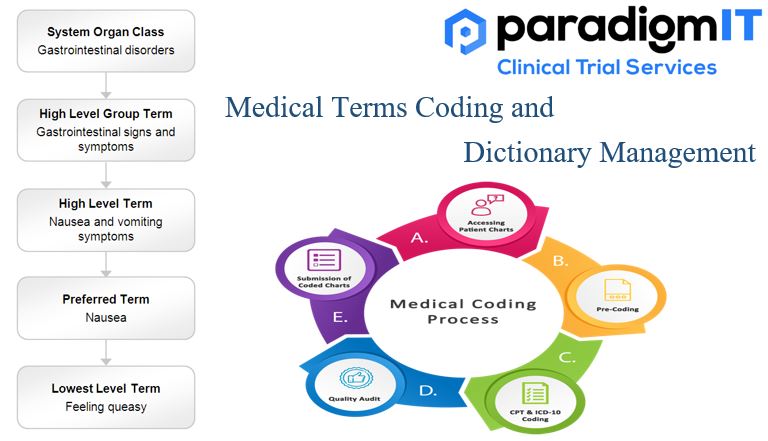
Effective communication might make the difference between timely treatment and potential difficulties in the ever-changing world of healthcare. Medical Term Coding and Dictionary Management are the unsung heroes of precision and clarity. These essential components not only simplify difficult medical terminology but also improve patient care and stimulate research innovation. Consider a scenario where a […]