Clinical trials are impactful only when we can draw meaningful conclusions from them. Interpreting clinical trial results is an essential task for healthcare professionals and patients alike. Understanding the findings can provide valuable insights into the safety, efficacy, and potential benefits or risks of a new medical intervention. Here are some key points to consider […]
Statistics and clinical research go hand in hand. Without adequate statistical support as a backbone, clinical trials will fail to deliver the expected results for far-fetched medical applications. Diligent efforts in statistical analysis will help researchers avoid common biases in clinical trials and translate the data sets into evidence for clinical decision-making. Statistical analysis, conducted […]
Clinical trials must include safety monitoring and adverse event reporting because they guarantee the health and safety of research participants. Depending on the nation and location, there are different regulatory criteria and best practices for safety monitoring and adverse event reporting, but they all typically work to ensure that clinical trials are run morally and […]
Data from clinical trials is extremely sensitive information, so participant security and privacy must be protected using strict protocols. Best practices for managing data privacy and security must be put in place due to the growing amount of clinical trial data being collected. In this post, we will look at a few recommended practices that […]
In the product development cycle of any medical device, human factors consideration can play a crucial role. It is also known as “Usability engineering” because it focuses on the possible interactions and interfaces between a medical device and its user. Though it may seem straightforward, human factors-based engineering can be complex at multiple levels. The […]
The FDA’s post-market surveillance program is designed to keep an eye on the efficacy and safety of pharmaceuticals and medical devices that have already received market approval. This is accomplished through an adverse event reporting system, which enables medical experts, patients, and customers to document any adverse reactions, side effects, or other issues connected to […]
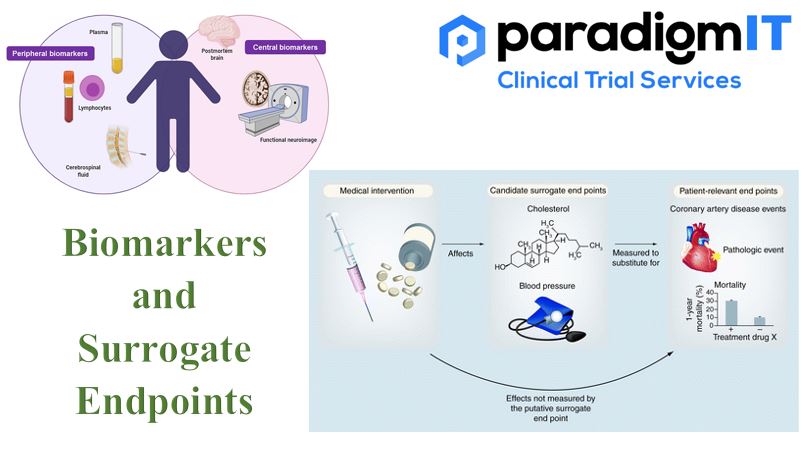
Bio-markers have versatile applications in healthcare, from diagnosis to prognosis. Apart from indicating the changes in physiologic and pathological health, they help us to understand the bodily response to different therapeutic interventions and measure the specific outcomes. As part of the clinical research continuum, biomarkers can help in the recruitment of clinical trial participants, the […]
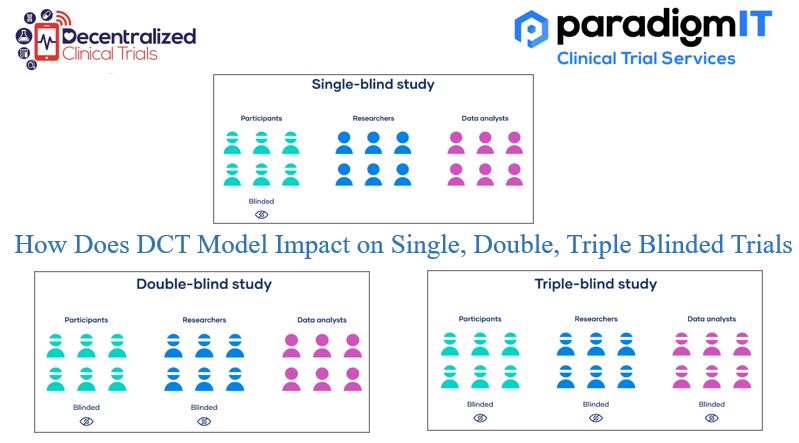
A clinical trial model known as “decentralized clinical trial” (DCT) makes use of technology to conduct some components of the experiment remotely from locations other than conventional clinical sites. DCTs may offer several benefits over conventional clinical trials, including improved patient recruitment and retention, cheaper trial costs, and speedier trial completion dates. DCTs, however, also […]
Orphan diseases are rare diseases that are less common in the frequency of occurrence but cause a significant burden on the health care systems. Even though there is no straightforward definition for rare diseases, each country has their definition. For instance, in the European Union, a disease that affects less than 1 in 2000 of […]
The Food and Drug Administration (FDA) is a regulatory agency of the United States Department of Health and Human Services. It is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, food supply, cosmetics, and products that emit radiation. Here are some […]