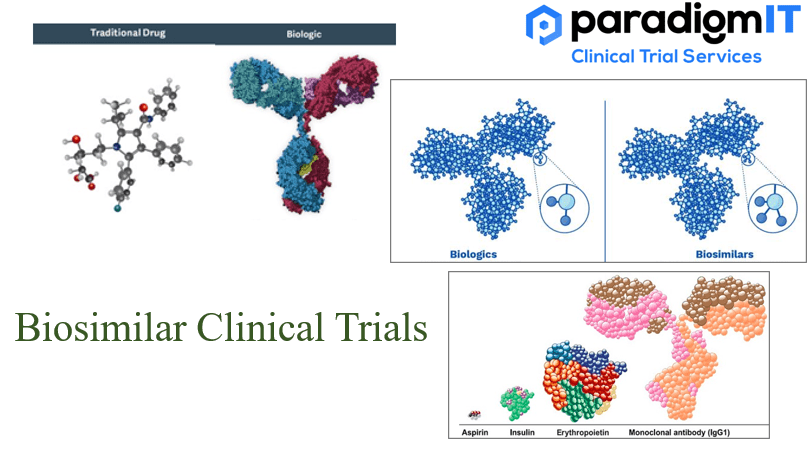
In the clinical setting, there is a growing body of evidence supporting the safety and efficacy of biosimilars. There are several biosimilars that have recently been approved or are in late-stage development for a variety of indications, such as cancer, autoimmune disorders, and inflammatory conditions. This is expected to increase the availability of biosimilars and expand treatment options for patients.
| Biologic Drugs | Biosimilar Drugs | Generic Drugs | Interchangeable Biosimilar |
| Medications that are generally made from natural sources/living organisms and developed using advanced science – are original biologic drug (reference product) | Medication that is already approved by FDA & are in the market. The criteria for the approval of a biosimilar drug are same as approved drugs, i.e., quality, efficacy, and safety. | Generics are same as Biosimilars but, chemically identical copies of branded (biological) drugs. However, Biosimilars are not identical copies of biological drugs. | It is that meets additional requirements like, a pharmacist may substitute an interchangeable biosimilar for its original drug without consulting the prescriber, depending on state pharmacy laws. |


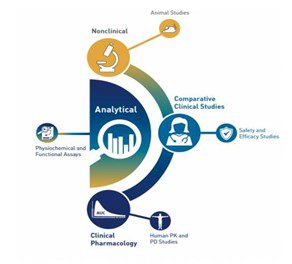
Biosimilar clinical trial includes:
- An Analytical Study – This study intends to demonstrate that the molecular pattern of the biosimilar is structurally and functionally identical to the reference drug.
- Animal Studies – The purpose of this process is to analyze the biosimilar drug’s toxicity.
- Clinical Pharmacology Studies – This action must be taken to show that the medication is high-quality, safe, and effective. There are also evaluations of the pharmacokinetic (PK) and pharmacodynamic (PD) effects.
- Additional Clinical Studies – A biosimilar development process aims to verify the high similarity between the biosimilar and reference product.

Biosimilar Review & Approval Process:
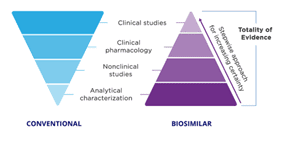
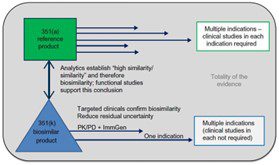
The process of making a biosimilar is intricate and multi-step & during each step, elements like the production cell line, the culture environment, and the formulation may modify the final product post-translational. Fewer steps are required to obtain FDA approval compared to the reference product, but this does not imply that the drug’s quality has been compromised in any way. The makers of biosimilars should provide comprehensive data packages that adhere to the exacting requirements established by the agency.
The FDA is focused on comparative testing and approvals and evaluates biosimilarity using a thorough methodology known as “Total of Evidence.” The organization advises the developers of biosimilar candidates to use a multi-step process, comparing the candidate to the biologic (reference product) at each stage, evaluating it for potential areas of residual ambiguity, and conducting studies intended to reduce those uncertainties.


Conclusion:
The use of biosimilars is on the rise, driven by the expiration of patents on many biologic drugs and increasing pressure to reduce healthcare costs. With a growing body of evidence supporting their safety and efficacy, and several biosimilars in late-stage development, it is expected that the availability of biosimilars will continue to expand, providing more treatment options for patients.
For more information –
Visit our website – www.paradigmit.com
Or you can write us at ask@paradigmit.com
Follow us for more – https://www.linkedin.com/company/paradigmittechnologyservices/?viewAsMember=true