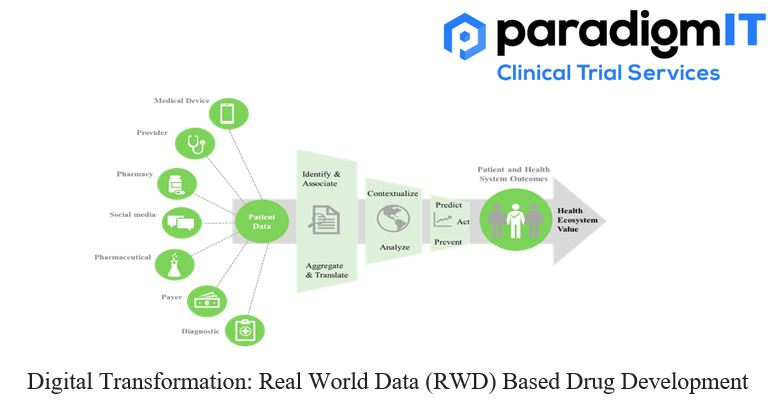
Personalized medicine is all set to transform the future of healthcare. With the advent of modern-day technology and tools, clinicians can now offer personalized-care and cater to the specific needs of their patients. To support physicians in this aspect, clinical researchers across the globe are tapping into the potential of real-world data. For instance, in […]
Drug Development has evolved by leaps and bounds over the past decade. As seen during the pandemic, the appropriate use of digital technologies has significantly accelerated the drug development process. Clinical researchers across the world are slowly adapting to this transition. Apart from the conventional sources of Clinical trial data, they are learning to depend […]
2023 is a crucial year for clinical research. On one side, we are coming out of a global pandemic. Another side, we are preparing for a wave of future uncertainties. The pandemic has directly changed the clinical trials processes. Patients are now at the centre of clinical trials. Other shifts in the industry are the […]
Clinical trials are research studies involving human subjects that evaluate the safety and efficiency of innovative therapies, medications, or medical devices. They are an essential stage in creating new medical treatments and play a significant role in deepening our understanding of human health and illness. Clinical trials in developing countries are an important aspect of […]
Data integrity: Data integrity refers to the accuracy, completeness, and consistency of data over its lifecycle, ensuring that it is not altered or destroyed in an unauthorized manner. In clinical trials, “data integrity” refers to the protection and preservation of the accuracy and reliability of the study data, from collection to analysis and reporting, to […]
Regenerative medicine Therapies are a burgeoning new therapy in the scientific field. A vast area that combines several scientific disciplines to speed up the healing process. It is described as replacing or regenerating human cells to restore the organ’s or tissue’s regular operation. It is a study of the body’s ability to heal itself by […]
The term “pharmacovigilance” in the digital era refers to the gathering, monitoring, and analysis of data on the safety of pharmaceuticals and medical devices using technology and digital platforms. This includes using social media, electronic health records, and other digital sources to identify and track potential drug side effects, as well as data analytics to […]
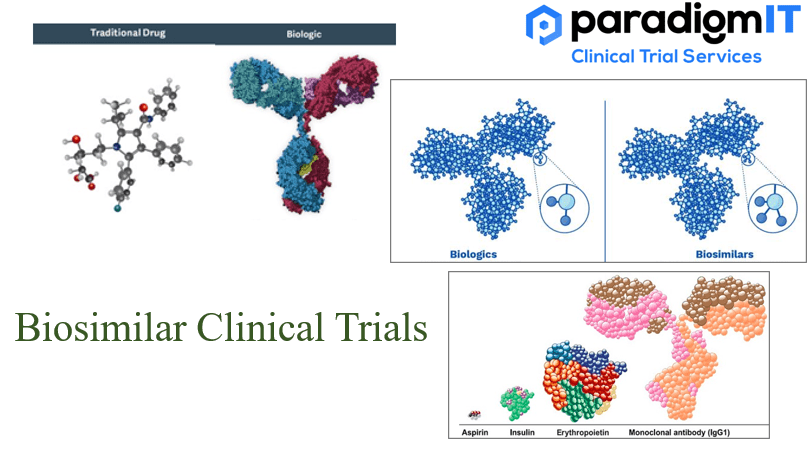
In the clinical setting, there is a growing body of evidence supporting the safety and efficacy of biosimilars. There are several biosimilars that have recently been approved or are in late-stage development for a variety of indications, such as cancer, autoimmune disorders, and inflammatory conditions. This is expected to increase the availability of biosimilars and […]
Our lives are now incredibly straightforward and structured, thanks to technology. Additionally, this technology has been essential in advancing clinical trials. Clinical trials are being digitized to save time, boost productivity, and reduce paperwork. In general discussion of Pre-Study Site Visits (PSSV) and Site Initiation Visits (SIV) in the clinical trial process, A PSSV is […]
The basic premise of search engine reputation management is to use the following three strategies to accomplish the goal of creating a completely positive first page of search engine results for a specific term…