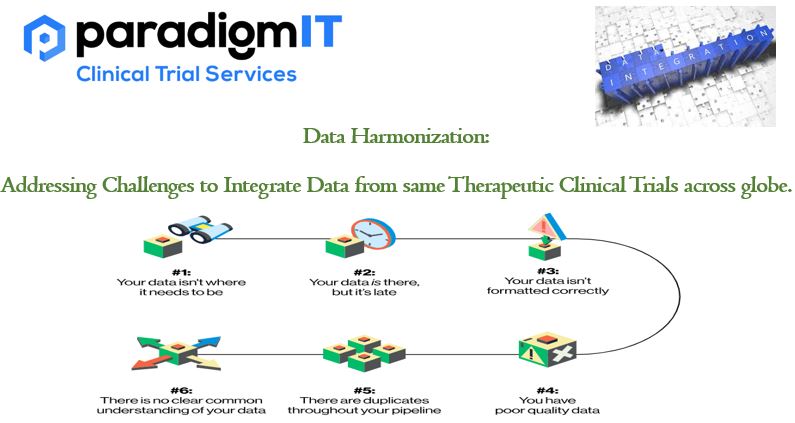
Due to several difficulties, integrating data from numerous clinical studies can be a challenging undertaking. Overcoming these obstacles to produce a consistent dataset that enables efficient analysis and interpretation is the process of data harmonization. Following are some typical issues in integrating data from several clinical studies and solutions to them:
Heterogeneous Data Structures: Clinical trial data architectures, variable names, and formats may differ. To harmonize the data, it is critical to map and standardize the variables across trials. This is accomplished by creating a single data model or data dictionary that defines the variables, their forms, and allowed values. The data dictionary ensures that different datasets are compatible and consistent with one another.
Inconsistent Data Interpretations: Endpoints, outcomes, and variables in clinical studies may all have different definitions. The protocols, case report forms, and study documentation must all be carefully examined to address this. Researchers can construct a set of definitions for variables and outcomes that are uniform by contrasting and aligning the definitions. Expert talks and initiatives to generate consensus may be involved.
Missing Data: In clinical trials, missing data is a common issue that varies depending on the research. When integrating data, it is vital to correctly manage missing data. Depending on the type and degree of missingness, a variety of procedures, including imputation and sensitivity analysis, can be used. Imputation techniques estimate missing values using observed data, whereas sensitivity analysis evaluates the effects of various missing data assumptions on study conclusions.
Varying Data Quality: Data quality in clinical trials might vary due to changes in data collection methods, errors made during data entry, or differences in study demographics. Data quality checks are performed as part of data cleansing and harmonization processes. This includes performing validation checks and cross-referencing with source documents to identify and resolve anomalies, inconsistencies, and conflicts.
Ethical and Regulatory Considerations: Compliance with ethical and regulatory requirements is necessary when combining data from various clinical trials. This entails safeguarding patient privacy and data protection, securing required authorizations, and adhering to pertinent laws like those governing informed consent and data sharing rules. To solve these issues, researchers should collaborate closely with ethics committees and data governance organizations.
Analytical Considerations: When analyzing data from multiple trials together, it may be required to consider study heterogeneity, different study designs, and statistical approaches. It is critical to account for these variables when undertaking combination analysis. Meta-analysis and mixed-effects modeling are two strategies that can be utilized to effectively address data diversity.
Various tactics can be used to address difficulties in combining data from numerous clinical trials. Consistency is improved by standardizing data structures and definitions using a shared data model or dictionary. Data gaps are handled by using methods like imputation or sensitivity analysis to handle missing data. Data accuracy is increased by carrying out quality checks and cleaning procedures. Compliance with privacy and consent laws is ensured by adhering to ethical and legal issues. The proper interpretation is ensured by taking into account study heterogeneity and various designs during analysis using methods like meta-analysis or mixed-effects modeling. For data harmonization to be successful, researchers, statisticians, and data managers must work together, have clear plans, and communicate openly.
For more information –
Visit our website – www.paradigmit.com
Or you can write us at ask@paradigmit.com
Follow us for more – https://www.linkedin.com/company/paradigmittechnologyservices/?viewAsMember=true