With the advent of modern-day tools, clinical trials are steadily transitioning into a technology-driven process. One such aspect that is changing is the traditional data collection process. Paper case report forms used over the past many years were quite handy, but they are also cumbersome, time-consuming, and prone to errors. The electronic Case Report form […]
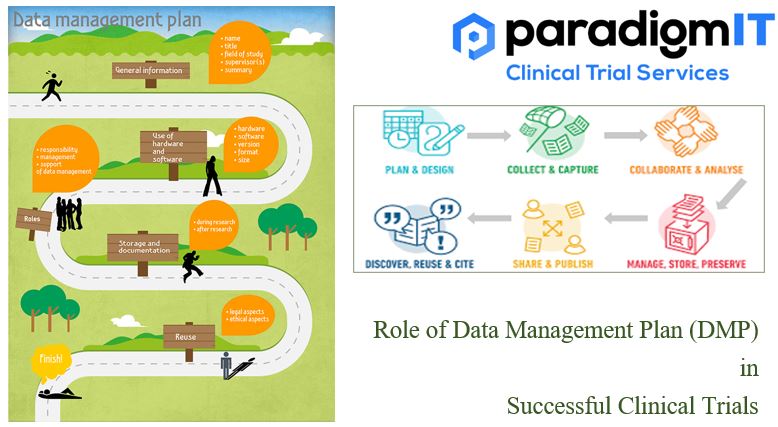
Clinical trials are complex and expensive undertakings, and the data collected during them is essential to ensuring their success. A well-written DMP can help to ensure that the data is accurate, complete, and compliant with regulatory requirements. What is a Data Management Plan (DMP)? A Data Management Plan (DMP) is a detailed and well-structured document […]
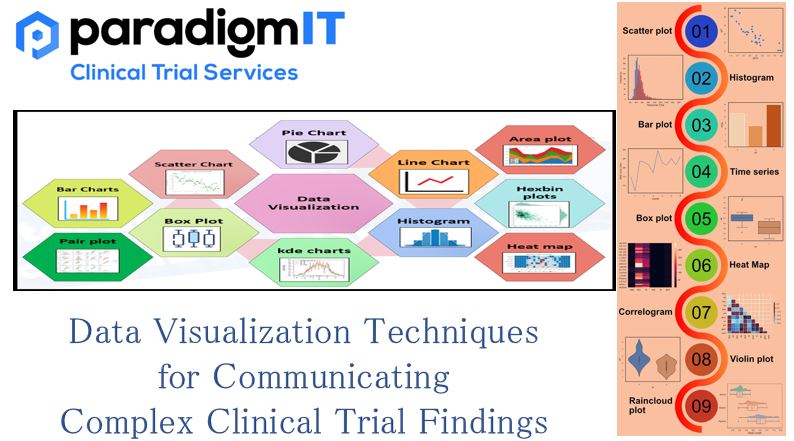
Understanding and deciphering clinical trial findings can be a complex challenge for the unprepared. With increasing data points, it is imperative to find better ways of data management and data analysis. To gain valuable insights from the available trial data, researchers may need to use the latest techniques and technologies in data visualization. Such techniques […]
Predication is the forecasting of future events based on previous similar patterns or models. Though it is not an easy task, science has always found a way to do it. Fred Hoyle (an English astronomer) put it crisply when he said, “Science is prediction, not explanation.” Technological advancement such as Artificial intelligence is transforming healthcare […]
Data collection at the site is the process of gathering data from a specific location or setting. This can be done in a variety of ways, such as surveys, interviews, observations, and document analysis. The process typically follows these steps: identify the data needs, design the data collection method, collect the data, analyze the data, […]
Data validation refers to the process of ensuring the accuracy and quality of data. It is implemented by building several checks into a system or report to ensure the logical consistency of input and stored data. Types of Data Validation Data Type Check A data type check confirms that the data entered has the correct […]
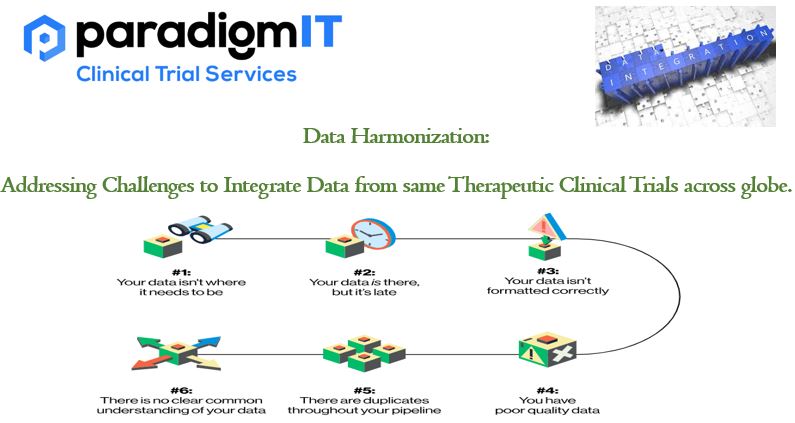
Due to several difficulties, integrating data from numerous clinical studies can be a challenging undertaking. Overcoming these obstacles to produce a consistent dataset that enables efficient analysis and interpretation is the process of data harmonization. Following are some typical issues in integrating data from several clinical studies and solutions to them: Heterogeneous Data Structures: Clinical […]
Data Mining in Clinical Trials Visit our website – www.paradigmit.com or write to us at ask@paradigmit.com In 2006, Clive Humby, a renowned British mathematician and entrepreneur in data science, coined the phrase, “Data is the new oil”. Two decades later, clinical researchers and data scientists are relating to this statement in a much more powerful […]
Clinical data management is essential for transforming raw data into insightful clinical knowledge. Ensuring the quality, integrity, and regulatory compliance of clinical data that requires gathering, organizing, validating, and analyzing the data. Effective clinical data management is essential for conducting successful clinical trials and generating reliable evidence to support medical decision-making. In this post, we’ll […]
Clinical trials are impactful only when we can draw meaningful conclusions from them. Interpreting clinical trial results is an essential task for healthcare professionals and patients alike. Understanding the findings can provide valuable insights into the safety, efficacy, and potential benefits or risks of a new medical intervention. Here are some key points to consider […]