Effective communication might make the difference between timely treatment and potential difficulties in the ever-changing world of healthcare. Medical Term Coding and Dictionary Management are the unsung heroes of precision and clarity. These essential components not only simplify difficult medical terminology but also improve patient care and stimulate research innovation.
Consider a scenario where a physician encounters a rare condition. With medical term coding and dictionary management, they swiftly access the relevant code for precise documentation, ensuring smooth interactions with insurance companies and reducing administrative burdens. This efficiency translates to improved patient care and satisfaction. Medical term coding and dictionary management, in addition to urgent patient treatment, feed medical research. These technologies use standardized codes to categorize and analyze patient data, allowing researchers to find trends, patterns, and treatment effects. This data-driven strategy speeds up breakthroughs and informs evidence-based medical practices. The medical term coding process is applicable in Clinical Trial Data and Healthcare Data collection.
Clinical Trial Data Collection:
Clinical trial data collection is the process of gathering information from trial participants. This information is used to assess the safety and efficacy of a new drug or treatment. Impact of not coding the terms in clinical trial data can have both pros and cons, each affecting various aspects of the trial process. It’s important to weigh these factors when deciding whether to code the data or not.
Pros:
- Cost Efficiency: Coding clinical trial data can be expensive and time-consuming. Not coding the terms can save money and resources.
- Accelerated Data collection: Not coding the terms can speed up the data collection process.
- Lightened Investigator Workload: Not coding the terms can reduce the workload on study investigators.
Cons:
- Difficulty in data analysis and interpretation: Without coding, clinical trial data is unstructured and difficult to analyze and interpret. This can make it difficult to identify trends and patterns in the data and draw meaningful conclusions from the trial.
- Increased risk of errors: When clinical trial data is not coded, there is a higher risk of errors in data entry and analysis. This can lead to inaccurate results and misleading conclusions.
- Reduced data quality: Uncoded clinical trial data is of lower quality than coded data. This is because it is more difficult to validate and standardize uncoded data.
- Limited data sharing and reuse: Uncoded clinical trial data is more difficult to share and reuse than coded data. This is because uncoded data is not as portable and accessible.
Systems operated for medical coding of trial data:
There are several systems operated for medical coding of trial data. Some of the most common systems include:
- Medical Dictionary for Regulatory Activities (MedDRA): MedDRA is a standardized medical terminology that is used to code adverse events and other medical information in clinical trials.
- World Health Organisation Drug Dictionary (WHO-DD): The WHO-DD used to code drugs and other pharmaceutical products in clinical trials.
- Systematised Nomenclature of Medicine Clinical Terms (SNOMED CT): SNOMED CT can be used to code a wide range of medical information in clinical trials.
These systems are used by medical coders to assign standardized codes to medical terms in clinical trial data. This makes the data more structured and easier to analyze and interpret. It is important to note that the benefits of coding clinical trial data outweigh the costs. Coding improves the quality and accessibility of data and makes it more useful for researchers and clinicians.
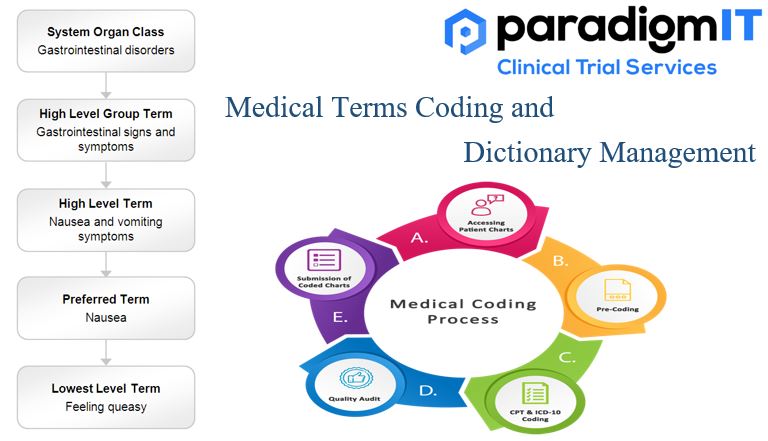
Medical Coding Process in Clinical Trials:
- Data Collection
- Medical Coding Systems Selection
- Identification of Medical Terms
- Assignment of Codes
- Quality Assurance and Validation
- Ongoing Data Management
- Regulatory Reporting
- Maintenance of Medical Coding Dictionary Updates
Healthcare Data Collection:
Decoding the Language of Medicine:
Medical Term Coding is transforming medical terminology into a global language. It entails tagging medical concepts, diagnoses, and treatments using standardized codes.
Empowering Healthcare with Dictionary Management:
Dictionary Management is at the foundation of effective medical word coding. This entails curating and updating massive databases of medical terminology, codes, and contextual meanings. A comprehensive dictionary becomes increasingly useful as medical knowledge grows. It provides reliable, up-to-date information to healthcare providers.
- ICD-10 (International Classification of Diseases, 10th Edition): is sometimes used in clinical research to classify and report medical diagnoses and conditions, particularly when aligning clinical trial data with healthcare records.
- ICD-10-PCS (International Classification of Diseases, 10th Edition, Procedure Coding System): ICD-10-PCS codes are used to classify medical procedures and interventions during clinical trials.
- LOINC (Logical Observation Identifiers Names and Codes): LOINC is used to standardize the coding of laboratory and clinical observations, measurements, and tests in clinical research settings.
- MeSH (Medical Subject Headings): been employed to index and catalog biomedical and health-related information, making it useful for organizing and retrieving medical literature relevant to clinical research.
- HCPCS (Healthcare Common Procedure Coding System): HCPCS codes may be used in clinical trials to document and classify certain healthcare procedures, services, and supplies, especially when billing and reimbursement are involved.
The medical coding process requires careful planning, training, and quality control to ensure the accuracy and consistency of coded data. Properly coded data is crucial for maintaining its integrity and ensuring the safety and well-being of trial participants.
For more information –
Visit our website – www.paradigmit.com
Or you can write us at ask@paradigmit.com
Follow us for more – https://www.linkedin.com/company/paradigmittechnologyservices/?viewAsMember=true